One Month In: Assessing the Impact of the Falsified Medicines Directive on the Global Healthcare Supply Chain
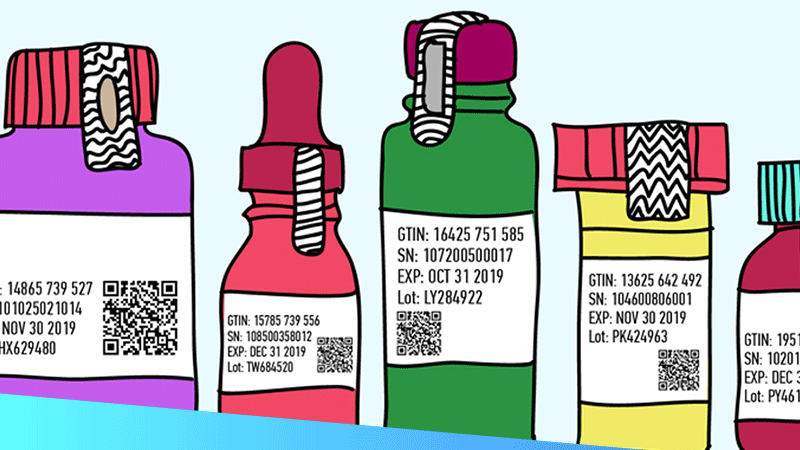
It has been just one month since European Directive 2011/62/EU – better known as the Falsified Medicines Directive (FMD) – went into effect across Europe. Yet the potential implications of such a measure, intended to prevent the introduction of illegal medicine into the legal supply chain, have been discussed for years by global pharmaceutical leaders. As Reuters recently explained:
“Drugmakers, wholesalers and pharmacies across Europe have worked for more than four years on a system based on a shared database and tamper-proof packages with barcodes … to fulfill the European Union’s Falsified Medicines Directive (FMD).”
And during this time the world was watching, curious about how such a directive would be put into practice. That is because the rise of counterfeit medication distribution is not an issue exclusive to the 400,000 European Union (EU) pharmacies that must now comply with the FMD. It is a global issue that, for decades, has been amplified by insufficient end-to-end traceability solutions and a lack of standardised oversight throughout pharma supply chains. The World Health Organization (WHO) first flagged their concern about the increasing number of counterfeit medicines in legal distribution networks in 1998. It proceeded to stand up the International Medical Products Anti-Counterfeiting Task Force (IMPACT) in 2006.
“Since then, IMPACT members have been collaborating closely on international criminal investigations, assisting countries in strengthening their own detection and enforcement systems, and working with industry to develop such measures as secure, high-tech pharmaceuticals packaging,” WHO explains in this brief.
But it wasn’t until the FMD took full effect last month that industry-wide changes were widely enforced. For example, there must now be “a unique identifier and an anti-tampering device on the outer packaging of medicines” and “strengthened record-keeping requirements for wholesale distributors.” And though the effectiveness of these now-obligatory safety features may not be clear quite yet, we can be sure their impact will be closely measured and scrutinised for years to come.
In fact, I am often asked if I think the Falsified Medicines Directive is going to work. (Since February, this question has increasingly shifted to: “Do you think FMD is working?”)
Each year, hundreds of thousands of deaths worldwide are attributed to the sale and subsequent consumption of counterfeit medicines. And there is certainly a risk of that number rising given that the WHO now values the global counterfeit drug market at around $200 billion annually. Though Europe is taking substantial, regulatory action to (hopefully) avert a public health crisis, exacting real change will take more than a single piece of legislation on one continent. It will also take more than a “wait and see” approach from other regions who are considering whether to follow the EU’s lead.
Yes, emulating the Falsified Medicines Directive across global regions could prevent fraudulent prescriptions from penetrating supply chains…
Though it’s too early to fully measure its effectiveness as a deterrent for counterfeiters, the multi-step, FMD-dictated medication dispensing process that pharmacies, hospitals and other medical facilities must now follow will certainly help the industry more easily identify fake medication and remove it from circulation.
But advocating for all stakeholders to proactively execute a technology-based attack plan could be just as effective at increasing safety and quality compliance on a global scale, if not more so.
Increasing the utilisation of mobile computing, barcoding scanning, labeling and RFID technologies that have been around for decades would easily enable those involved in the manufacturing and distribution of medications to “walk the walk.” Supply chain organizations will have the tools to take meaningful, measurable action to minimise the risk of counterfeit medicines entering into circulation.
The pharma supply chain is complex. There are a lot of touchpoints for a single pill, ranging from raw material suppliers to manufacturers to warehouse operators, distributors, retail pharmacies. There are also many different doctors, nurses, technicians and pharmacists involved in the ordering, management and dispensing of medicines at the patient level in clinical healthcare and pharmacy settings.
Fortunately, the same 2D barcode scanning technologies used to comply with FMD requirements during the final prescription inspection and authentication phase can be utilised to enforce stricter quality control measures from the raw materials source to the factory and throughout the distribution process. It does not matter if the measures are self-imposed by supply chain organizations or defined by regulators. The effectiveness of these scanning technologies in managing the movement of inventory across multiple industries (including pharma), controlling the quality of goods and services and protecting patients has long been proven. In fact, there would be a great deal of secondary benefits gained by proactively enacting standards – and employing scanning technologies – similar to those in the FMD to reduce the risk of falsified medicine distribution, including:
- Manufacturing quality: You can create a complete record of every product as it moves through every step of your manufacturing facility and document the results of quality control inspections to increase accountability and compliance and address potential issues in real time.
- Warehouse and distribution control: You maintain up-to-the-minute insight into the location of your medication, along with confirmation of who has handled the product while in transit. This is valuable should investigations be required into whether or not counterfeit medications entered into circulation via your supply chain.
- Patient safety: It becomes easier to check the accuracy and quality (i.e. date) of a prescription, which is already common practice.
- Pharmacy stock management: You gain real-time visibility into prescription stock, reduce manual data entry requirements and gain accurate unit-dose level data for all products. This aids with decommissioning expired or damaged stock and reverse decommissioning of uncollected medication.
- Patient verification and care: You will have the tools and data sets to generate patient-specific information to improve the quality and speed of care.
There are also a host of other technologies, ranging from rugged handheld, tablet and vehicle-mounted computers to label printers and sensors to IoT and RFID technologies such as Zebra Savanna™ and Zebra SmartLens™, that can be employed by workers at your various supply chain touchpoints to reduce the risk of falsified medicines making their way into the fold.
This collection of enterprise-grade mobile, IoT and sensing technology can also be used to mitigate fear surrounding medication shortages, whether the result of potential Brexit-related actions impacting pharma supply chains governed by the FMD or the result of past supply versus demand miscalculations.
The data collectively generated and distributed by these devices in the factory, warehouse, distribution center or healthcare/pharmacy setting can be quickly aggregated and analysed to gain an accurate understanding of current raw material and in-stock medication inventory. Manufacturers and their partners can then reconcile supply against current demand and adjust their sourcing and production levels accordingly to prevent shortages (or costly overstocks). In fact, it will be difficult for global pharma leaders to be effective and remain agile in their fight against falsified medicines without these technologies.
It is not enough to rid the system of the dangerous drugs; they have to remain capable of replacing them with the quality, often lifesaving, prescriptions that consumers depend on every day. The industry cannot risk disrupting patient care in the process of improving that care. Fortunately, it doesn’t have to thanks to the many technology solutions available today to help maintain continuity of legitimate pharmaceutical production and distribution.
Final note:
There is no reason why pharma supply chain organizations should wait for politicians to insist on improved medicine traceability practices to invest in technologies that facilitate better business practices. Protecting your customers – whether they are the healthcare providers writing the prescriptions, the pharmacies dispensing them, or the patients themselves – should be incentive enough to make an investment in technologies that simultaneously improve materials handling, quality control, inventory oversight and stock management. Protecting your brand’s reputation, and eliminating the possible degradation of value for your products, comes with the increased visibility and control you gain over your products from the moment prescription ingredients are sourced to the moment a legal, quality prescription is consumed by the patient.
Visit our Falsified Medicines Directive resource page to learn more about the solutions available to help you manage all phases of medication production and distribution with confidence, from inventory control to prescription verification and accurate dispensing.

Wayne Miller
Wayne is the Zebra lead for Healthcare solutions and works to directly improve patient care, supporting partners and end users. Delivering the latest solutions that assist hospitals and healthcare organizations to deliver efficiencies and improve patient care and outcomes. Wayne has worked for Zebra Technologies for 17 years and has a true passion for healthcare, technology and innovation.






