Transform retail operations with Zebra’s retail technology solutions, featuring hardware and software for improving inventory management and empowering teams.
Streamline operations with Zebra’s healthcare technology solutions, featuring hardware and software to improve staff collaboration and optimise workflows.
Enhance processes with Zebra’s manufacturing technology solutions, featuring hardware and software for automation, data analysis, and factory connectivity.
Zebra’s transportation and logistics technology solutions feature hardware and software for enhancing route planning, visibility, and automating processes.
Zebra's hospitality technology solutions equip your hotel and restaurant staff to deliver superior customer and guest service through inventory tracking and more.
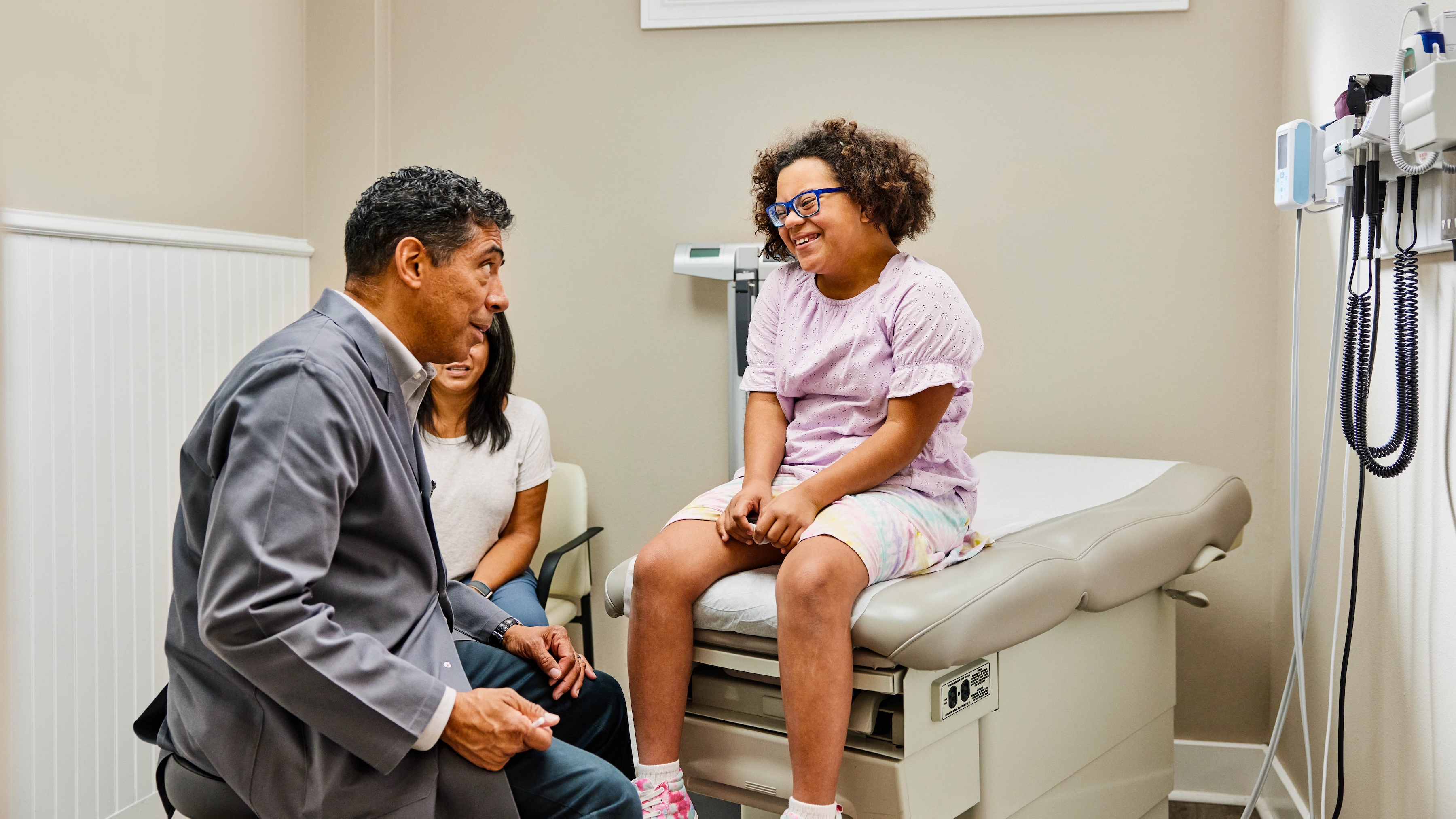
Zebra's market-leading solutions and products improve customer satisfaction with a lower cost per interaction by keeping service representatives connected with colleagues, customers, management and the tools they use to satisfy customers across the supply chain.
Empower your field workers with purpose-driven mobile technology solutions to help them capture and share critical data in any environment.
Zebra's range of mobile computers equip your workforce with the devices they need from handhelds and tablets to wearables and vehicle-mounted computers.
Zebra's desktop, mobile, industrial, and portable printers for barcode labels, receipts, RFID tags and cards give you smarter ways to track and manage assets.
Zebra's 1D and 2D corded and cordless barcode scanners anticipate any scanning challenge in a variety of environments, whether retail, healthcare, T&L or manufacturing.
Zebra's extensive range of RAIN RFID readers, antennas, and printers give you consistent and accurate tracking.
Choose Zebra's reliable barcode, RFID and card supplies carefully selected to ensure high performance, print quality, durability and readability.
Zebra's rugged tablets and 2-in-1 laptops are thin and lightweight, yet rugged to work wherever you do on familiar and easy-to-use Windows or Android OS.
With Zebra's family of fixed industrial scanners and machine vision technologies, you can tailor your solutions to your environment and applications.
Zebra’s line of kiosks can meet any self-service or digital signage need, from checking prices and stock on an in-aisle store kiosk to fully-featured kiosks that can be deployed on the wall, counter, desktop or floor in a retail store, hotel, airport check-in gate, physician’s office, local government office and more.
Discover Zebra’s range of accessories from chargers, communication cables to cases to help you customise your mobile device for optimal efficiency.
Zebra's environmental sensors monitor temperature-sensitive products, offering data insights on environmental conditions across industry applications.
Zebra's location technologies provide real-time tracking for your organisation to better manage and optimise your critical assets and create more efficient workflows.
Enhance frontline operations with Zebra’s AI software solutions, which optimize workflows, streamline processes, and simplify tasks for improved business outcomes.
Empower your frontline with Zebra Companion AI, offering instant, tailored insights and support to streamline operations and enhance productivity.
The everything you need to rapidly and cost effectively develop high-performance AI vision applications on Zebra mobile computers.
Zebra Workcloud, enterprise software solutions boost efficiency, cut costs, improve inventory management, simplify communication and optimize resources.
Keep labour costs low, your talent happy and your organisation compliant. Create an agile operation that can navigate unexpected schedule changes and customer demand to drive sales, satisfy customers and improve your bottom line.
Drive successful enterprise collaboration with prioritized task notifications and improved communication capabilities for easier team collaboration.
Get full visibility of your inventory and automatically pinpoint leaks across all channels.
Reduce uncertainty when you anticipate market volatility. Predict, plan and stay agile to align inventory with shifting demand.
Drive down costs while driving up employee, security, and network performance with software designed to enhance Zebra's wireless infrastructure and mobile solutions.
Explore Zebra’s printer software to integrate, manage and monitor printers easily, maximising IT resources and minimising down time.
Make the most of every stage of your scanning journey from deployment to optimisation. Zebra's barcode scanner software lets you keep devices current and adapt them to your business needs for a stronger ROI across the full lifecycle.
RFID development, demonstration and production software and utilities help you build and manage your RFID deployments more efficiently.
RFID development, demonstration and production software and utilities help you build and manage your RFID deployments more efficiently.
Zebra DNA is the industry’s broadest suite of enterprise software that delivers an ideal experience for all during the entire lifetime of every Zebra device.
Advance your digital transformation and execute your strategic plans with the help of the right location and tracking technology.
The Zebra Aurora suite of machine vision software enables users to solve their track-and-trace, vision inspection and industrial automation needs.
Zebra Aurora Focus brings a new level of simplicity to controlling enterprise-wide manufacturing and logistics automation solutions. With this powerful interface, it’s easy to set up, deploy and run Zebra’s Fixed Industrial Scanners and Machine Vision Smart Cameras, eliminating the need for different tools and reducing training and deployment time.
Aurora Imaging Library™, formerly Matrox Imaging Library, machine-vision software development kit (SDK) has a deep collection of tools for image capture, processing, analysis, annotation, display, and archiving. Code-level customisation starts here.
Aurora Design Assistant™, formerly Matrox Design Assistant, integrated development environment (IDE) is a flowchart-based platform for building machine vision applications, with templates to speed up development and bring solutions online quicker.
Designed for experienced programmers proficient in vision applications, Aurora Vision Library provides the same sophisticated functionality as our Aurora Vision Studio software but presented in programming language.
Aurora Vision Studio, an image processing software for machine & computer vision engineers, allows quick creation, integration & monitoring of powerful OEM vision applications.
Adding innovative tech is critical to your success, but it can be complex and disruptive. Professional Services help you accelerate adoption, and maximise productivity without affecting your workflows, business processes and finances.
Zebra's Managed Service delivers worry-free device management to ensure ultimate uptime for your Zebra Mobile Computers and Printers via dedicated experts.
Find ways you can contact Zebra Technologies’ Support, including Email and Chat, ask a technical question or initiate a Repair Request.
Zebra's Circular Economy Program helps you manage today’s challenges and plan for tomorrow with smart solutions that are good for your budget and the environment.
Ready-To-Use Indicators
HEATmarker VVM
Since 1996, HEATmarker® Vaccine Vial Monitors (VVM), manufactured by Temptime®, have been used to monitor the temperature exposure of vaccines, as recommended by the WHO and UNICEF. In 2012, WHO determined VVM to be a "critical characteristic", a required element of all vaccine tenders submitted to UNICEF.
Monitor the Cumulative Temperature Exposure of Vaccines
HEATmarker VVM and VVM+ indicators are affixed to a vaccine vial by the manufacturer to allow for full-life temperature monitoring. The inner square features a heat-sensitive material that becomes darker when exposed to heat. The colour change is faster at higher temperatures and slower at lower temperatures. At the end point, the inner square is the same colour as or darker than the outer circle. This allows the HEATmarker VVM to give health workers an overview of the cumulative heat exposure of the vaccine and a clear visual indication when it has reached its time-temperature end point, informing them whether the vaccine should be administered.
Simple Identification of Cumulative Heat Exposure
Simple Identification of Cumulative Heat ExposureSimple Identification of Cumulative Heat Exposure
HEATmarker VVM inner square features a heat-sensitive chemistry that shows a gradual and permanent colour change. Initially light in colour, it becomes darker when exposed to heat. At the end point, the inner square is the same colour as, or darker than the outer circle. This visual indication alerts healthcare workers of the heat exposure of each vaccine vial to inform them whether the vaccine should be administered.
Preserve Valuable Inventory
Preserve Valuable InventoryPreserve Valuable Inventory
Since their appearance changes gradually over time, health workers can use HEATmarker VVM to easily monitor heat exposure and dispose of only the vaccine inventory that has reached their temperature endpoints. In addition, they can prioritise the use of those vaccines that have had more heat exposure (without exceeding the limit) over those that have not.
Extend Vaccinations to the Last Mile
Extend Vaccinations to the Last MileExtend Vaccinations to the Last Mile
The colour change of HEATmarker VVM provides a visible and reliable indicator allowing healthcare professionals to confidently carry vaccines to previously unreachable populations.
Menu

- HEATmarker VVM 250
- HEATmarker VVM 30
- HEATmarker VVM 14
- HEATmarker VVM 11
- HEATmarker VVM 7
- HEATmarker VVM 2

HEATmarker VVM 250
HEATmarker VVM 250 is optimal for use with very high stability vaccines, as defined by WHO/PQS/E006/IN05.3. It features a maximun time to end-point of 250 days at 37°C. Shelf life is 5 years.

HEATmarker VVM 30
HEATmarker VVM 30 is optimal for use with high stability vaccines, as defined by WHO/PQS/E006/IN05.3. It features a maximun time to end-point of 30 days at 37°C. Shelf life is 5 years.

HEATmarker VVM 14
HEATmarker VVM 14 is optimal for use with medium stability vaccines, as defined by WHO/PQS/E006/IN05.3. It features a maximun time to end-point of 14 days at 37°C. Shelf life is 5 years.

HEATmarker VVM 11
HEATmarker VVM 11 is optimal for use with intermediate stability vaccines, as defined by WHO/PQS/E006/IN05.3. It features a maximun time to end-point of 11 days at 37°C. Shelf life is 4 years.

HEATmarker VVM 7
HEATmarker VVM 7 is optimal for use with moderate stability vaccines, as defined by WHO/PQS/E006/IN05.3. It features a maximun time to end-point of 7 days at 37°C. Shelf life is 4 years.

HEATmarker VVM 2
HEATmarker VVM 2 is optimal for use with vaccines with the least stability, as defined by WHO/PQS/E006/IN05.3. It features a maximun time to end-point of 2 days at 37°C. Shelf life is 4 years.
Additional Resources
| HEATmarker VVM Fact Sheet R2, Doc #3189 | Download Fact Sheet Download Fact Sheet | |
|---|---|---|
| WHO/PQS/E006/IN05.4 Compliance | Learn More Learn More | |
| WHO Testing Protocol | Learn More Learn More | |
| WHO-UNICEF VVM Policy Statement | Learn More Learn More | |
Legal Terms of Use Privacy Policy Supply Chain Transparency
ZEBRA and the stylized Zebra head are trademarks of Zebra Technologies Corp., registered in many jurisdictions worldwide. All other trademarks are the property of their respective owners. Note: Some content or images on zebra.com may have been generated in whole or in part by AI. ©2025 Zebra Technologies Corp. and/or its affiliates.